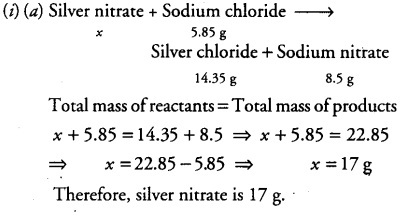
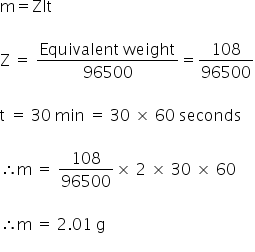What mass of silver nitrate will react with 5.85 g of sodium chloride to produce 14.35 g of silver chloride & 8.5 g of sodium nitrate if the conservation of mass is true?
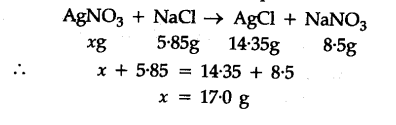
What mass of silver nitrate will react with 5.85 g of sodium chloride to produce 14.35 g of silver chloride and 8.5 g of sodium nitrate, if the law of conservation of

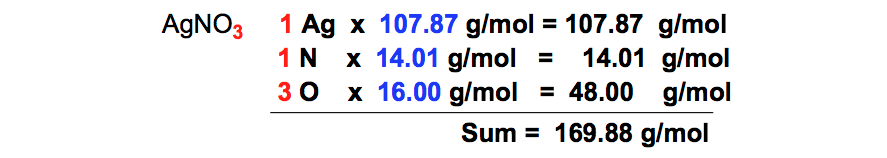
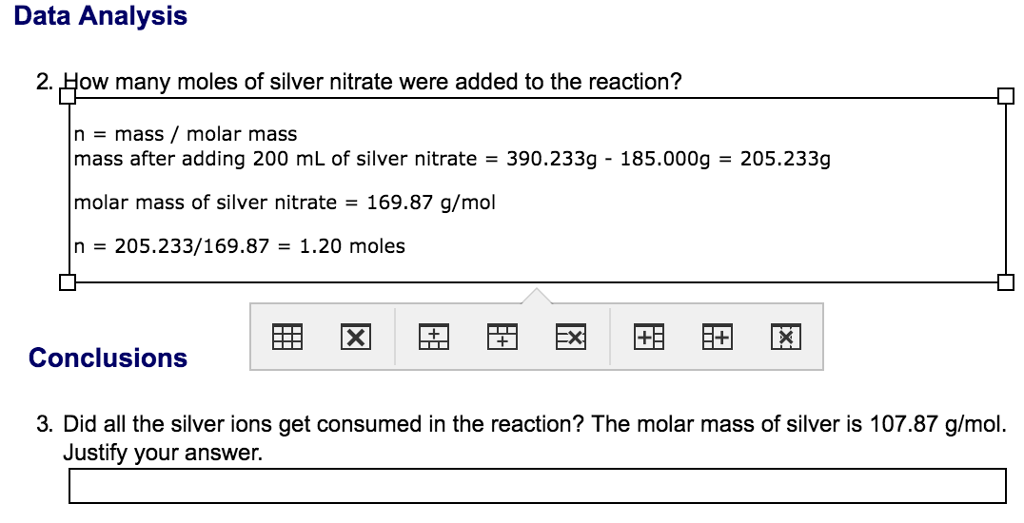
SOLVED: Stoichiometry What mass of silver chloride, AgCl; precipitates if 5.00 g of silver nitrate, AgNO3,is added t0 a sodium chloride solution? AgNO3 NaCl AgCl NaNO3 If0.162 g of aluminum is burned

what mass of silver nitrate is needed to prepare 100cm3 of silver nitrate solution, concentration - Brainly.com

Question Video: Calculating the Mass of Solute Needed to Prepare a Solution with a Desired Concentration and Volume | Nagwa

The value of the observed and calculated molecular weight of silver nitrate is 92.64 and 170, respectively. The degree of dissociation of silver nitrate is:

When 9.65 coulomb of electricity is passed through a solution of silver nitrate (Atomic mass of Ag = 108 g mol^(-1), the amount of silver deposited is :

The balanced equation shows that two moles of silver nitrate react with one mole of copper - International Baccalaureate Chemistry - Marked by Teachers.com

CHEMICAL QUANTITIES. Measuring Matter Count Weigh Volume Each of these measurements is related to a single quantity called the “mole” - ppt download
10 g NaCl solution is mixed with 17 g of silver nitrate solution. Calculate the weight of silver chloride precipitated.AgWO_{3} NNOHto to ggCl+NaNO_{3} [Ag=1081] | Snapsolve
Solved] what mass of silver could be plated onto a spoon from electrolysis of silver nitrate with a 3.50-A current for 45.0 minutes? | Course Hero