Calculate radius of second Bohr radius of hydrogen atom and then also calculate the speed of the electron and total - Sarthaks eConnect | Largest Online Education Community

Find the value of angular velocity of an electron revolving in first Bohr orbit - Physics - Atoms - 3664251 | Meritnation.com
41. The velocity of an electron in a certain bohr orbit of H atom is 1/275th of velocity of light . What is the value of n for the orbit?

a) The electron in the first Bohr orbit of a hydrogen atom travelling... | Download Scientific Diagram

The velocity of an electron in the first Bohr orbit of hydrogen atom is `2.19 xx 10^(6)ms^(-1)`. - YouTube
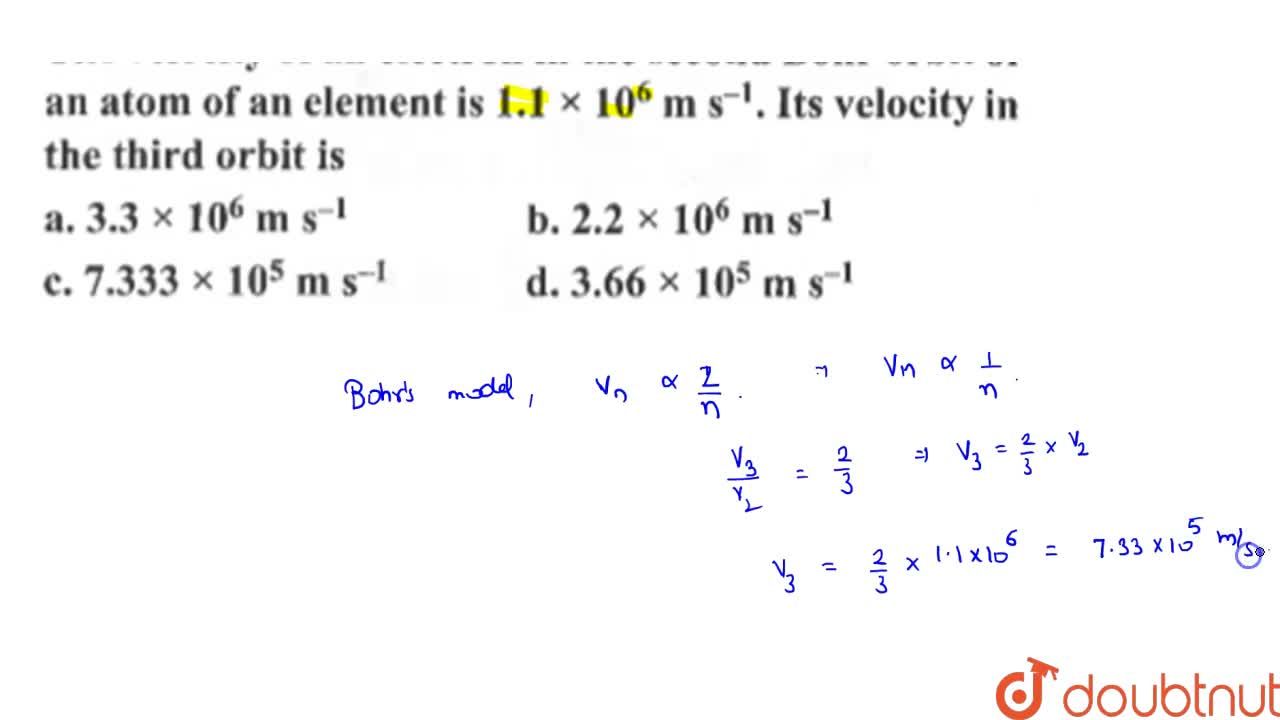
The velocity of an electron in the second Bohr orbit of an element is 1.1 xx 10^(6) s^(-1) Its velocity in the third orbit is

By what factor the velocity of an electron in a Bohr's orbit for a hydrogen atom will change, if the principal quantum number is doubled:

The velocity of an electron in the second Bohr orbit of an element is `1.1 xx 10^(6) s^(-1)` - YouTube

The circumference of the first Bohr orbit in H atom is 3.322 × 10^-10m . What is the velocity of the electron of this orbit?
Calculate velocity of electron in first Bohr orbit of hydrogen atom (Given r = a0). - Sarthaks eConnect | Largest Online Education Community
Velocity of electron in the nth bohr orbit of hydrogen like atom is given by 2.18×10raise to power 8Z/n cm/s the velocity of electron in the first bohr orbit of hydrogen atom ? (
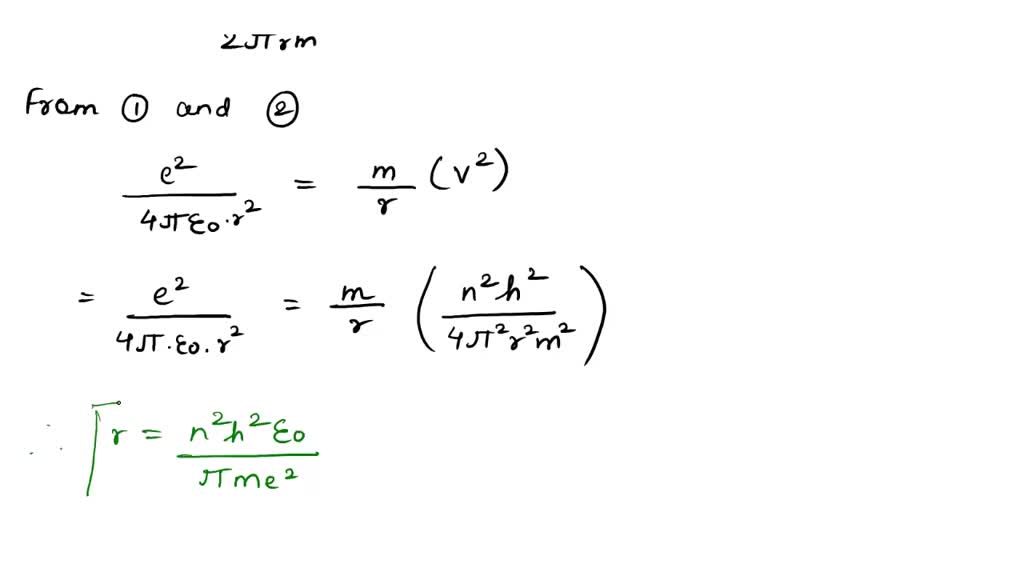
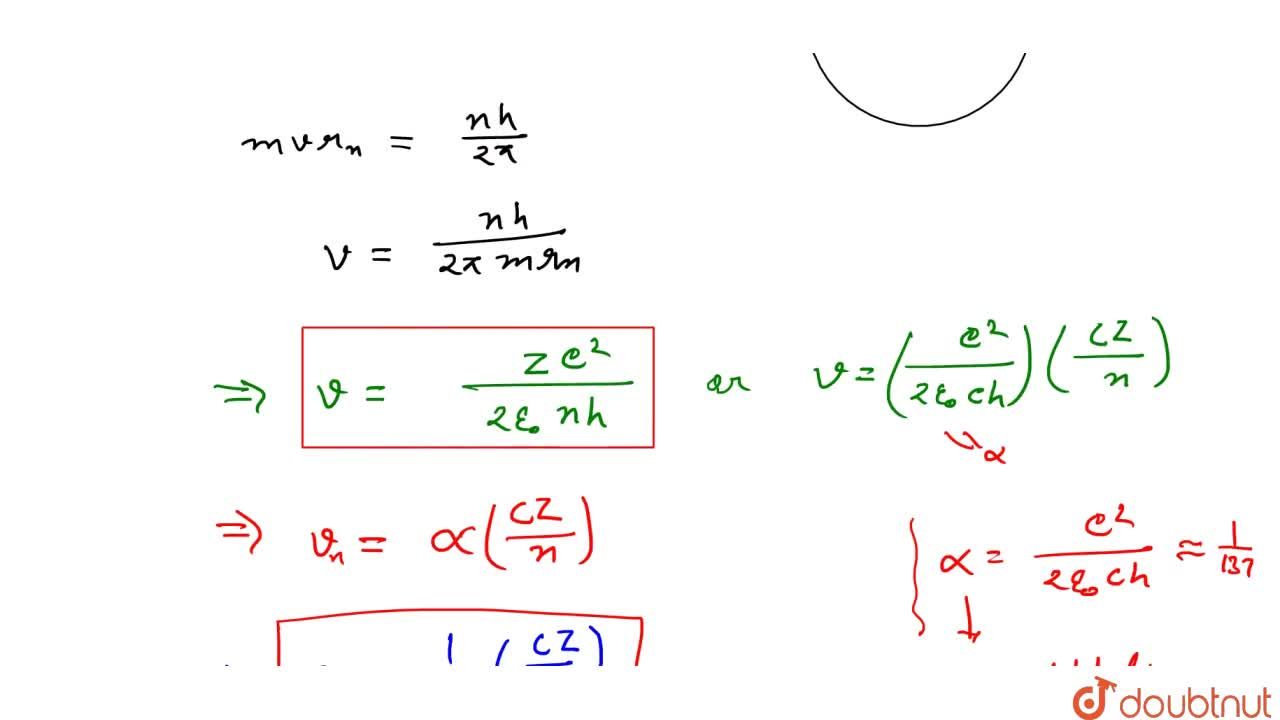
SOLVED: (a) Derive an expression for the electron's speed in the nth Bohr orbit (b) Prove that the orbit with highest speed is the n = 1 orbit, with U1 ke? /h.




.PNG)
